Papers
Collections
- 2023 - Faraday Discussion - Astrochemistry at high resolution
- 2022 - Phys. Chem. A - 10 Years of the ACS PHYS Astrochemistry Subdivision
- 2013 - Chem. Rev. - Astrochemistry Issue
The molecular universe
- A.G.G.M. Tielens, Rev. Mod. Phys. 85, 1021 (2013)
I. Introduction
This section starts with the role of molecules in space.
- molecules are abundant and widespread in space
- formation of stars and evolution of galaxies
- periotic interstellar molecules
mightrepresent the first step towards life.
Then the developments of how to detect molecules in space are briefly stated.
- 1930s: the discovery of diatomic molecules in space through their optical transitions.
- 1960s: simple molecules through rotational transitions
- 1990s: molecules are present all of space, infrared and submilllimeter observation
- 1995, the Infrared Space Observatory
- 2005, the Spitzer Space Telescope
- 2009, the Herschel Space Observatory
- ..., the Atacama Large Millimeter Array
- who knows, the James Webb Space Telescope
II. The molecular inventory of the interstellar medium
Interstellar medium is where the further generations of stars are created.
- ISM plays a central role in the evolution of galaxies.
- PAHs are the most abundant polyatomic species in space, but no individual PAH has been identified.
- How is the first generation of star created?
- Why is
kineticsmore important thanthermodynamicsin determining the organic inventory in space?
Selected papers
- [2021 - ApJ - Germán Molpeceres and Johannes Kästner Computational Study of the Hydrogenation Sequence of the Phosphorous Atom on Interstellar Dust Grains]
- Water ice is the most abundant solid in the Universe. [2016 - PCCP - Martin R. S. McCoustra et.al.]
- H2 and H2O are the most abundant molecule and solid phase molecular constituent in interstellar dense clouds, respectively.
- [2017 - Space Sci. Rev. - Cuppen et.al]: Grain Surface Models and Data for Astrochemistry
- [2021 - ACS Earth Space Chem. - Jorge O. Oña-Ruales et.al.]: The Helicenes: Potential Carriers of Diffuse Interstellar Bands
- [2021 - ACS Earth Space Chem. - Jan H. Bredehöft et.al.]: Electron-Induced Processing of Methanol Ice
- The electron irradiation of CH3OH ice -> radicals and ionized,protonated-CH3OH -> CH4, H2CO, HOCH2CH2OH, CH3OCH2OH, CH3OCH3, CH3CH2OH.
- The disadvantage of IR is that it is hard to distinguish compounds with similar functional groups.
Glycine
# The most stable conformer of glycine import ssl ssl._create_default_https_context = ssl._create_unverified_context from ase.data.pubchem import pubchem_atoms_search atoms = pubchem_atoms_search(cid=750) atoms.center() atoms.write('glycine.xyz', plain=True)
H2
- The smallest and most abundant molecule in the universe
- The key to star formation and the starting point for the gas-phase chemistry of ISM
C2H2
- J.H. Cacy et al. ApJ 1989, 342, L43-L46: First gas phase detection of acetylene in the ISM by IR observations from the NASA IRTF 3m telescope
- F. Lahuis and E.F. van Dishoeck A&A 2000, 355, 699-712 ISO-SWS spectra of gas phase C2H2 and HCN toward massive young stellar objects
2020 - Nat Astron - PH3
- J.S. Greaves, A.M.S. Richards, W. Bains, P.B. Rimmer, H. Sagawa, D.L. Clements, S. Seager, J.J. Petkowski, C. Sousa-Silva, S. Ranjan, E. Drabek-Maunder, H.J. Fraser, A. Cartwright, I. Mueller-Wodarg, Z. Zhan, P. Friberg, I. Coulson, E. Lee, and J. Hoge, Nat. Astron. 1 (2020).
- https://www.nature.com/articles/s41550-020-1174-4
PH3 is thought to be a biosignature, since the production of PH3 on Earth is often related with the activities of microorganisms. In addition, PH3 gets easily oxidized under Venus's atmosphere and is unstable. The confirmation of PH3 on Venus's atmosphere means that there must be a stable mechanism to generate PH3.
- It is difficult to detect PH3 outside Earth, because PH3's spectra features are strongly absorbed by Earth's atmosphere.
- The existence of PH3 is not the evidence of life.
- The formation pathway of PH3 is still unknown.
2020 - Nat Astron - An experimental study of the surface formation of methane in interstellar molecular clouds
- D. Qasim, G. Fedoseev, K.-J. Chuang, J. He, S. Ioppolo, E.F. van Dishoeck, and H. Linnartz, Nat Astron 1 (2020).
- https://www.nature.com/articles/s41550-020-1054-y
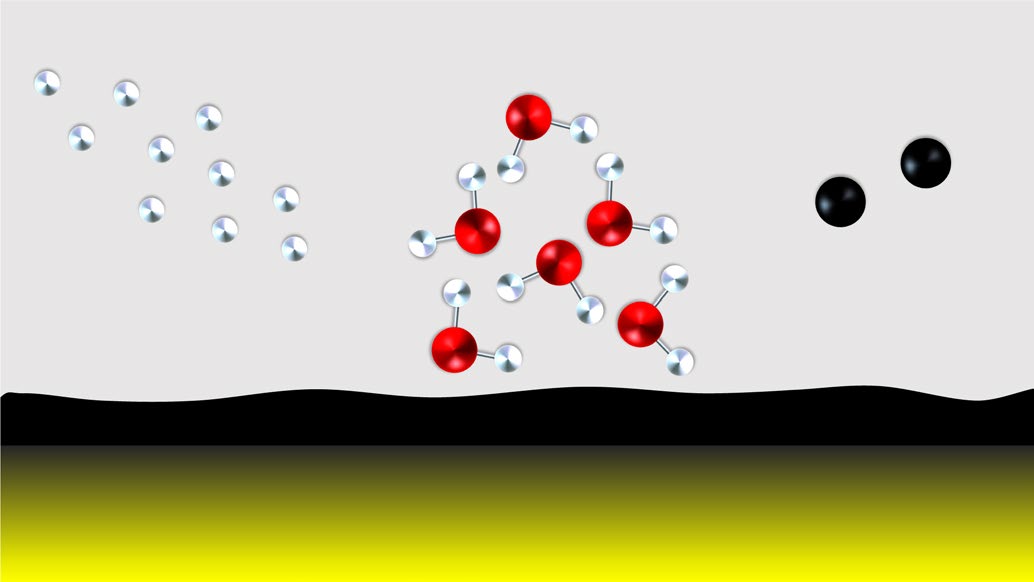
This paper is the first experimental proof of solid-state CH4 formation by atomic C and H under conditions mimicking those of interstellar molecular cloud environments. It starts with the existence of CH4 in space and interstellar environments where CH4 is detected. Experimentalists have tried to reproduce interstellar environments in laboratories to reveal how CH4 is formed in space. The new part of this work is that it solves a technical issue which is the combination between an atomic carbon source and a ultrahigh vacuum (UHV) set-up. This special design is necessary to study atom-induced surface reactions under molecular cloud conditions.
What is the guess for CH4 formation from observational point of view?
CH4 is expected to be formed by the hydrogenation of C on dust grains, and CH4 ice is strongly correlated with solid H2O.
What systems are investigated in laboratories and what are found by experiments?
Three layers structure: Species/Surface/Substrate
| Species | Surface | Substrate |
|---|---|---|
| atomic C + atomic H | amorphous carbon (carbonaceous surface) | Inert gold substrate (10 K) |
| atomic C + atomic H + H2O | amorphous carbon (carbonaceous surface) | Inert gold substrate (10 K) |
- CH4 is the main product from experiments.
- $\text{CH}_n$ radicals and their recombination products such as C2H4, C2H4, C2H6 are not detected.
- The author's explanation is that recombination between $\text{CH}_n$ radicals and atomic H is efficient and the life time of $\text{CH}_n$ radicals is short in their experiment environment
-
There are no citations or further description of this statement. Does this mean the recombination between $\text{CH}_n$ radicals and atomic H is easier than the recombination between $\text{CH}_n$ radicals and $\text{CH}_n$ radicals?
- The producing rate of CH4 is higher when H2O is included.
How to understand the role of H2O?
The Abundance of C2H4 in the Circumstellar Envelope of IRC+10216
- J.P. Fonfría, K.H. Hinkle, J. Cernicharo, M.J. Richter, M. Agúndez, and L. Wallace, ApJ 835, 196 (2017).
- C2H4 relative abundance to H2: ~ 10^-8
- up to 25% of the C2H4 molecules at 20R* could condense onto dust grains